A previous cancer diagnosis can preclude patients from consideration for solid organ transplantation. Statistical models may improve candidate selection. We fitted statistical cure models and estimated 5-year cancer-specific survival (5yCSS) for colorectal cancer patients in the United States using registry data. The median cure probability at cancer diagnosis for patients in the general population was 0.67.
Transforming the lives of people with cancer and organ transplants through integrated healthcare and research.

Watch intro video (4 min)
The Center for Innovations in Cancer & Transplant was founded with the following goals:
- To provide outstanding multidisciplinary clinical care for pre- and post-transplant patients with cancer.
- To lead exceptional, multidimensional, patient-focused research of cancer before and after organ transplant through a robust bioregistry and collaborative research network.
We seek to do this through a first-of-its kind clinic, a new bioregistry, and our own original research.
Learn more about the Center
Clinic
The first-of-its-kind multidisciplinary consult clinic for organ transplant candidates and recipients with cancer.
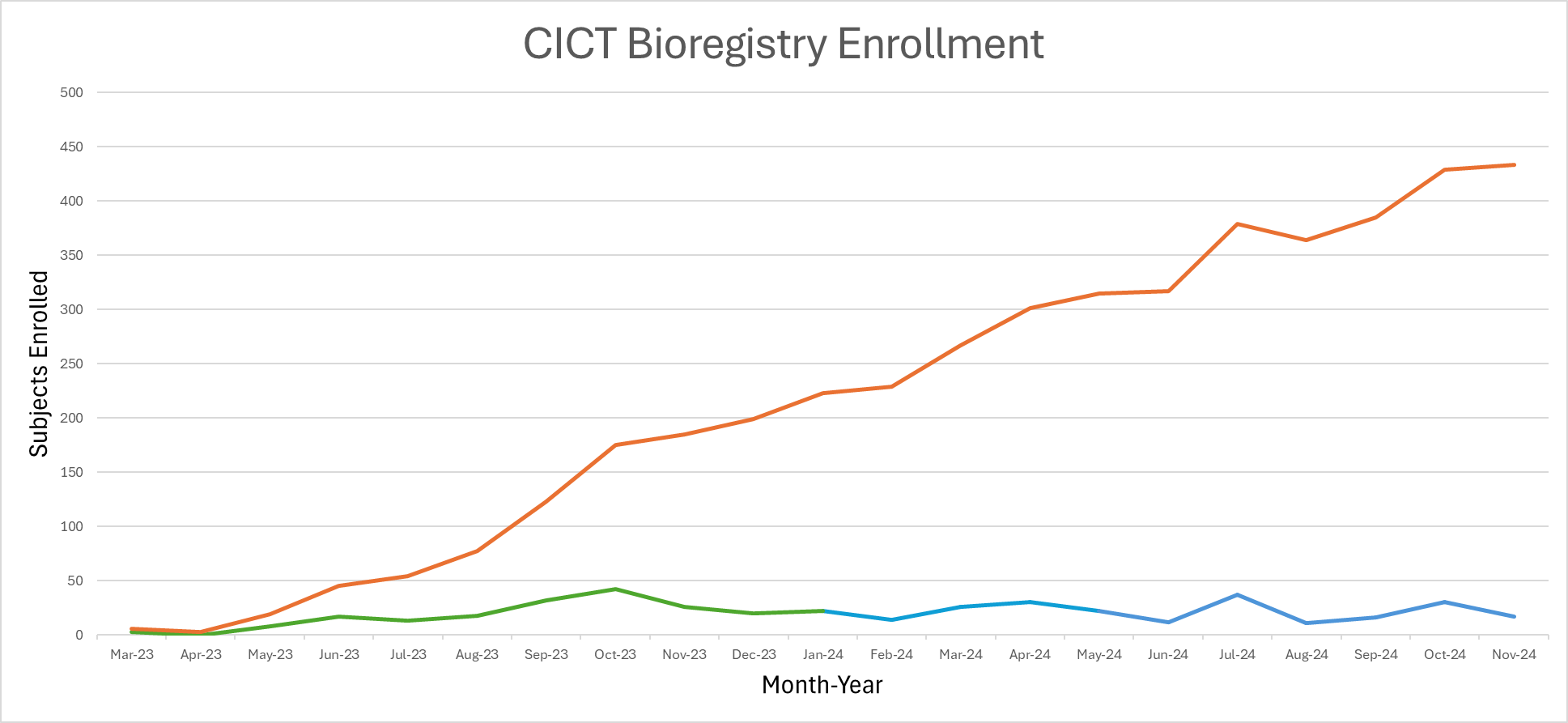
Bioregistry
A registry to integrate patient data from cancer centers and transplant programs around the world.
Research
Investigating the immune mechanisms and epidemiology of cancer with solid organ transplantation.
Recent publications
Cure models, survival probabilities, and solid organ transplantation for patients with colorectal cancer
Eric A Engels… Christopher D Blosser…Ruth M Pfeiffer
American Journal of Transplantation
Original Work
DOI: 10.1016/j.ajt.2024.08.018
PMID: 39182612
Among 956 colorectal cancer patients who underwent solid organ transplantation, the median time since diagnosis was 6.3 years and the median 5yCSS at transplantation was 0.96. Patients with a 5yCSS below 0.90 had increased posttransplant cancer-specific mortality (hazard ratio 3.31, 95% CI 1.52-7.21). Compared with recently published guidelines, our models suggested shorter wait times for some groups of colorectal cancer patients (eg, stage IIA cancers) and longer wait times for others (stages IIB, IIIB, IIIC, IV). In conclusion, colorectal cancer patients undergoing solid organ transplantation had excellent prognoses, reflecting selection incorporating existing guidelines and clinical judgment. Nonetheless, 5yCSS probabilities estimated from cure models offer additional prognostic information for patients considered for transplantation and identify situations where current guidelines might be revised. We developed a web-based tool for clinicians to calculate 5yCSS probabilities for use in transplant evaluation for individual colorectal cancer patients
Scope and Consistency of Cancer Outcomes Reported in Randomized Trials in Kidney Transplant Recipients
Eric H Au…Christopher D Blosser…Jonathan C Craig
Kidney International Reports
8(2):274-281
Original Work
DOI: 10.1016/j.ekir.2022.10.032
PMID: 36815120
Cancer is an important outcome in kidney transplantation, but the scope and consistency of how cancer is defined and reported in trials involving kidney transplant recipients has not been evaluated. This study aimed to assess the range and variability of cancer outcomes in trials involving kidney transplant recipients.
The ClinicalTrials.gov database was searched from February 2000 to July 2021 to identify all randomized controlled trials (RCTs) in adult kidney transplant recipients, and which included cancer as a specified outcome. The definition of cancer, types of cancer (if any), timepoint(s) of measurement and method of aggregation were extracted for each cancer outcome.
Of the 819 trials in kidney transplantation, only 84 (10%) included 1 or more cancer outcomes. Of these, 72 of 84 (86%) trials included cancer as a secondary outcome and 12 of 84 (14%) considered cancer as a primary outcome. The most frequent description of cancer was “malignancy” (n = 44, 43%), without reference to diagnostic criteria, histology, grade, or stage. The 2 most common cancer types were posttransplant lymphoproliferative disorder (PTLD) (n = 20, 20%) and nonmelanoma skin cancer (n = 10, 10%). Several methods of aggregation were identified, including incidence or rate (n = 47, 46%), frequency or proportion (n = 30, 29%), and time to event (n = 5, 5%). Approximately half the cancer outcomes were measured at a single time point (n = 44, 52%).
Cancer is an infrequently reported outcome and is inconsistently defined in trials of kidney transplant recipients. Consistent reporting of cancer outcomes using standardized definitions would provide important information on the impact of cancer in patients after kidney transplantation.
Evaluation of the Modified Oxford Score in Recurrent IgA Nephropathy in North American Kidney Transplant Recipients: The Banff Recurrent Glomerulonephritis Working Group Report
Nada Alachkar…Christopher D Blosser…Serena M Bagnasco
Transplantation
107(9):2055-2063
Original Work
DOI: 10.1097/TP.0000000000004640
PMID: 37202854
The modified Oxford classification mesangial and endocapillary hypercellularity, segmental sclerosis, interstitial fibrosis/tubular atrophy, and the presence of crescents (MEST-C) of immunoglobulin A nephropathy (IgAN) was recently shown to be a predictor of graft failure in Asians with recurrent IgAN. We aimed to validate these findings in a cohort from North American centers participating in the Banff Recurrent Glomerulopathies Working Group.
We examined 171 transplant recipients with end-stage kidney disease because of IgAN; 100 of them with biopsy-proven recurrent IgAN (57 of them had complete MEST-C scores) and 71 with no recurrence.
IgAN recurrence, which was associated with younger age at transplantation ( P = 0.012), strongly increased the risk of death-censored graft failure (adjusted hazard ratio, 5.10 [95% confidence interval (CI), 2.26-11.51]; P < 0.001). Higher MEST-C score sum was associated with death-censored graft failure (adjusted hazard ratio, 8.57 [95% CI, 1.23-59.85; P = 0.03] and 61.32 [95% CI, 4.82-779.89; P = 0.002] for score sums 2-3 and 4-5 versus 0, respectively), and so were the single components endocapillary hypercellularity, interstitial fibrosis/tubular atrophy, and crescents ( P 0.05).
Our findings may validate the prognostic usefulness of the Oxford classification for recurrent IgAN and support the inclusion of the MEST-C score in allograft biopsies diagnostic reports.